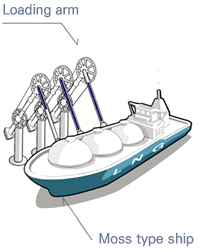
KOGAS
Natural Gas Extraction and Refining
Natural gas is a naturally-occurring combustible gas comprised mainly of hydrocarbons. There are many stages to exploiting natural gas, e.g., exploration, drilling, verification of presence of gas, mining, and supply
Freshly extracted natural gas contains contaminants such as water, high molecular mass hydrocarbons, nitrogen, helium, carbon dioxide, and hydrogen sulfide in addition to the main components methane (approx. 90%), ethane, propane, and butane. If these substances are used without refinement, the heating value and physicochemical properties will vary. In order to increase value as a fuel, and to use natural gas as a valuable resource, it is made into a clean fuel through the separation and refining process as follows.-
Moisture Removal
Moisture must be removed during the liquefaction of natural gas as it can condense and cause serious damage to the facility. After removal, 96?127g of water remains per 10m3 of gas at 15°C and 1 atm (absolute pressure).
-
Removal of Sulfur Compounds
Mined natural gas contains a significant amount (more than 5 g/m3) of sulfur compounds (H3S), and these sulfur compounds are pollutants. The sulfur is removed by using amines that selectively absorb it (sweetening process). The separated sulfur compounds are then used as raw materials to produce sulfur and sulfuric acid.
-
Carbon Dioxide Removal
Extracted natural gas consists of up to 45% carbon dioxide (CO2) by volume. A large amount of carbon dioxide has a great effect on heating value like nitrogen, so it is removed through the natural gas liquefaction process.
-
Removal of Dirt and Oil
Dust refers to the sand that is discharged when natural gas is extracted from underground reservoirs, and the dust from machinery wear and tear. This dust can cause major damage to the impellers of pumps and compressors that rotate at high speed. In addition, the oil lubricant injected into the machines disperses in fine particles when there is a sudden pressure change, and this mixes with the natural gas, thereby lowering purity. This dust and oil are therefore usually removed with cyclones, electrostatic precipitation, or oil scribing.
Liquefaction Plant

Natural gas extracted and refined from production sites is converted into a liquid state through a liquefaction process for long-distance transportation, or to store a large amount of natural gas in a small storage space.
Natural gas in a liquefied state is called liquefied natural gas (LNG), and this is easily transported and stored since it is reduced 600-fold in volume. The main component of natural gas is methane, and the liquefaction temperature of methane is very low, so it is difficult to liquefy this with typical equipment. Natural gas is therefore liquefied through the following special methods.-
Expansion Method (Turbo Expander Cycle)
This method exploits the principle that when natural gas is pressurized and then rapidly adiabatically expanded with a turbine, its temperature drops rapidly, and it becomes liquid.
-
The Cascade Cycle
This is a method of liquefying natural gas by sequentially lowering its temperature with various refrigerants. First, ethylene is used to liquefy propane, and this liquefied propane is then used as a refrigerant to liquefy natural gas.
-
Mixed Refrigerant Cycle (Multi-Component Refrigerant Cycle)
In this process, if a suitable mixture of hydrocarbons and nitrogen is used as a refrigerant and undergoes condensation and expansion processes, un ultra-low temperature sufficient to liquefy natural gas can be obtained.
Storage Tank Facility in Producing Country
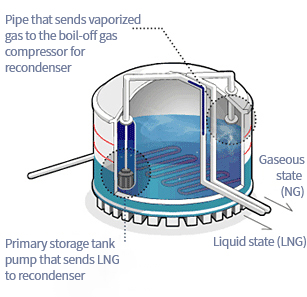
The storage tanks in the producing and importing countries are structurally the same, but those in the producing countries are only used for temporary storage of liquefied natural gas before it is loaded on a transport ship.
The structure of the storage tank is like a giant flask with a double wall. The outer wall is made of special post-stressed concrete, which safeguards against all forces to which the tank is subjected; this corresponds to a flask’s outer bottle. The inner wall is a sealed membrane wall made of stainless steel in a special shape to store liquid natural gas. In order to store liquefied natural gas safely, the space between the walls is filled with polyvinyl chloride foam, which has excellent thermal insulation properties, and by preventing heat from penetrating the tank, this prevents the natural gas from being vaporized.Loading Dock